 In a post on Using Point-of-Care Testing in the Physician’s Office, we discussed the advantages of performing routine lab testing in a Physician Office Lab (POL), rather than sending out samples to centralized laboratories.
In a post on Using Point-of-Care Testing in the Physician’s Office, we discussed the advantages of performing routine lab testing in a Physician Office Lab (POL), rather than sending out samples to centralized laboratories.
Performing lab tests in your office helps avoid one of the few still-unaddressed-inefficiencies in patient healthcare: the sending out of samples and then waiting for results to come in.
But for many doctor’s offices there is concern and uncertainty surrounding the time, costs and process of making the switch to in-house moderately complex testing .
Many physician office Point-of-Care laboratories begin in-house testing with CLIA-waived tests, or tests that are considered simple tests with a low risk of an incorrect result. At some point, however, a physician’s office lab may decide to upgrade their testing to offer additional in-house assays for the clear reasons outlined in our earlier post on Point-of-Care Testing in POLs.
 Understanding Test Complexity
Understanding Test Complexity
CLIA (Clinical Laboratory Improvement Amendments, a law administering the certification & oversight of clinical laboratory testing) organizes tests into one of three categories: Waived, Moderate Complexity and High Complexity. Each category has its own testing site requirements, which grow more stringent at higher levels.
When categorizing an assay, the FDA looks at:
- Whether interpretation of results and ‘independent judgment’ will be needed
- How much quality control and calibration of lab instruments will be required
- The amount of training the test will demand to ensure successful instrument operation
- The complexity of the test procedures and methods.
In general, the tests a physician’s office sends out to a hospital, central or reference lab are typically considered Moderately Complex.
Deciding to Upgrade to Moderately Complex Testing
The decision to offer moderately complex tests in-house is often driven by one or both of the following factors:
- A test is currently sent out, necessitating an additional patient visit, a delay in treatment, higher costs and more healthcare provider & patient time.
- Certain tests are high volume, and offer revenue-generating opportunities for the physician’s practice.
Whatever the reason – and there are many – upgrading to moderately complex testing in-house can offer tremendous benefits, from better & faster patient care and additional revenues to patient & treating physician peace of mind. Many physician owned labs see this as an excellent trade-off for the time and money that may be needed to upgrade from a waived to a moderately complex lab.
Not As Complex As You Might Believe
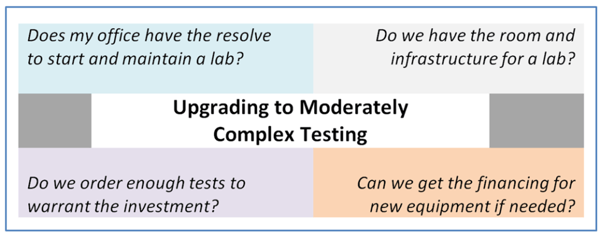
The complexity of upgrading to a moderately-complex lab is often overstated. In fact, when properly-implemented, becoming a Moderately Complex Lab isn’t as difficult as physician offices may think and the requirements tend to be less onerous than physicians likely believe.
The decision to upgrade, however, will come down to SWOT and cost/benefit analyses which should be conducted to determine if the physician’s office can support the lab.
Labs wanting to upgrade their testing capabilities have some justified concerns:
- Is it cost-effective or revenue-generating?
- Is it feasible from a workflow and work space perspective?
- Can I efficiently (and with as little fuss as possible) meet the requirements of this upgrade?
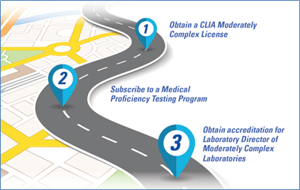
Upgrading to a Moderately Complex Testing Lab: The Process
The process required in order to upgrade from a waived to a Moderately Complex testing lab takes time and effort, and may incur cost. Here’s a broad outline of the steps involved in upgrading to a moderately complex testing regimen:
- Upgrade your certificate to a Certificate of Compliance
File a change in certificate type and pay certification fees, as needed. - Meet personnel standards for moderately complex facilities
These standards include laboratory training, previous experience, or CMEs covering laboratory topics. One option – which has proven popular among Sekisui’s FastPack IP users – is to utilize the University of Iowa’s CLIA Online Certification Course. - Participate in an approved Proficiency Testing (PT) Program
A Centers for Medicare and Medicaid Services (CMS) approved PT program will evaluate your lab’s accuracy with interpretation and judgment. A list of PT providers can be found at: cms.gov. - Apply appropriate QA and QC standards
Your lab will need to establish and follow a quality control program, perform Method Verification at installation, and adhere to instrument maintenance schedules as required by the manufacturer.
While requirements must be met and may seem like a significant burden, it can – in many cases - be well worth the investment of time and effort.
There’s Help Available to Evaluate Upgrading Your POL
It is important to ensure your laboratory testing supplier can meet all of your volume and test type needs, has the equipment you need and provides a high level of subject matter expertise to support your POL operations.
In fact, providers such as Sekisui Diagnostics can help. Sekisui has assisted numerous labs in making the transition from a CLIA-waived lab to a Moderately Complex lab using the ready-to-go FastPackTM system. Our subject matter experts can help guide you on decision analysis, approval process and instrumentation.
Want to learn more about upgrading your POL to Moderate Complexity testing? The CMS.gov website is an excellent resource for answering questions on the process.


Share Article