PAMA—What is it? Does it matter to me, you may ask? Unfortunately, no, we’re not talking about the pomegranate liqueur made by PAMA Spirits (though a discussion of yet another piece of government legislation impacting healthcare may make you wish we were!).
The Protecting Access to Medicare Act of 2014 (PAMA) was signed into law on April 1, 2014. It establishes a market-based pricing system for lab tests in an effort to bring Medicare payments into closer alignment with private payer rates. The goal of PAMA is to collect data around clinical diagnostic laboratory tests (CDLTs) and advanced diagnostic laboratory tests (ADLTs) to determine what level of payments will be provided for these tests.
When Terra Firma Feels More Like Quicksand
PAMA raised the collective blood pressure of many lab communities—in 2018, year one of PAMA, 996 CPT codes (about 88 percent of all the lab CPT codes) experienced a reduction to meet the pricing mandate. And that wasn’t the only change. Medicare previously paid for CDLTs under the Clinical Laboratory Fee Schedule (CLFS), which provided payment for approximately 1,300 CDLTs. Below, the difference between the CLFS and PAMA are highlighted.
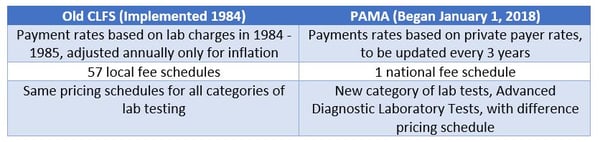
Unfortunately for labs, reductions to most CPT codes are expected in each of the first three years of implementation of PAMA. For now, reductions are capped at 10 percent annually. In 2019, year two, nearly 1,000 CPT codes are expected to be reduced by up to 10 percent again. Reimbursement data is being collected by labs in 2019 and the next changes to CLFS rates based on these findings is expected in 2021.
What’s New
In addition to these changes, PAMA also created a new category of lab tests called ADLTs, or advanced diagnostic laboratory tests. Tests that meet criteria for this category will have separate pricing that requires reporting of payer reimbursements annually. To be considered an ADLT test, it must be performed by a single lab and either be approved by the FDA, or be a test that evaluates a patient’s DNA, RNA, or proteins; provide new clinical diagnostic information that cannot be obtained from any other test; and use a unique algorithm that predicts the chance of a patient developing a condition or responding to a treatment.
PAMA also eliminated the discounts applied to tests frequently ordered together in panels—the Automated Test Panel reimbursement schedule. While the panels continue to exist and are paid at the “panel” rate, the tests within the panels are no longer discounted if ordered separately.
Survival is Not Guaranteed
It seems harsh and perhaps premature to say that not all labs are going to survive in the wake of PAMA, but that seems to be the general consensus of lab professionals. The American Clinical Laboratory Association (ACLA) in May 2018 described PAMA as “undermining the nation’s clinical laboratory infrastructure” and called the implementation “flawed and misguided.” According to the ACLA, the Department of Health and Human Services cherry-picked data from fewer than one percent of labs to determine rates for the CFLS. This flawed implementation has led to reduced and limited service offerings, forced changes to laboratory business models, and resulted in a reduction in the workforce.
Both the lab community and the general healthcare community seem to agree that the PAMA cuts are too steep; may reduce access to lab tests, especially in rural areas; and did not equitably align new payment rates because only a fraction of labs submitted data. But not all agree with that assessment—in late 2018, the U.S. Government Accountability Office (GAO) issued a report stating that the new rate calculation methodology and approach to bundled tests could cause Medicare to pay higher rates than in the past, costing as much as $10.3 billion from 2018 to 2020.
The GAO recommended that CMS make data collection more complete with data from more laboratories, re-set CLFS payment rates and rate reductions based on actual Medicare payment rates, and re-establish the bundled test panel reimbursement system.
The American Association for Clinical Chemistry (AACC) believes that laboratories serving nursing homes (thus receiving the majority of their lab testing payment from Medicare and Medicaid) will be the hardest hit—going so far as to say that many of these labs will not survive. Smaller labs in general will face a tough road.
Finding the Positive
Reading this probably makes you feel like you’ve turned on cable news or logged onto Twitter—bad news 24/7, right? Is there any upside to this massive sweep of changes? The AACC believes that labs that offer proprietary testing will fare best; and the new category of tests, ADLTs, are expected to get favorable pricing. For physician office labs, it’s likely to be hit or miss. Some of the most commonly performed waived tests will see increases in the next three years, but many other high-volume tests will see decreases in Medicare payments.
Google “How to survive PAMA” and you’ll get several pages of tips and strategies. It remains to be seen if “survival of the fittest” will be true in this case, but it certainly never hurts to improve your business model. The AACC recommends strong financial management with the ability to fully capture critical data and revenues, peak operational efficiency, and diversification. Ultimately, the full impact of PAMA on labs and patients is murky at best.



Share Article