
Ever since the U.S. Food and Drug Administration approved the first hormone therapy drug in 1942, hormone therapy has experienced twists and turns in the eyes of women, the public and scientific community. At one time the most prescribed treatment for menopausal symptoms in the United States, hormone therapy fell out of favor in the mid-1970s, recovered somewhat, then took a nosedive in 2002. Let’s reflect.
A 1966 book – “Feminine Forever” -- by gynecologist Robert Wilson, M.D., led to explosive growth of Premarin, a mix of estrogens derived from the urine of pregnant horses, but it also signaled “the start of a vexed relationship for women and hormone therapy,” writes the author of a February 2023 New York Times article.1 “The book was bold for its time, in that it recognized sexual pleasure as a priority for women,” she writes. “But it also displayed a frank contempt for aging women’s bodies and pitched hormones in the service of men’s desires.” By the mid-1970s, Premarin was the fifth-most prescribed drug in the United States.
In 1975, a research study published in the New England Journal of Medicine showed that menopausal women who took conjugated estrogens (i.e., a mixture of estrogen hormones) had an increased risk of endometrial cancer.2
Prescriptions dropped until researchers realized that they could all but eliminate the increased risk by prescribing progesterone, a hormone that inhibits the growth of cells in the uterus lining. Prescriptions climbed, and by the late 1990s, some 15 million women a year were receiving a prescription for it. Such was the case until July 9, 2002.3
The Bombshell
In 1991, the National Institutes of Health had started the Women’s Health Initiative to study health outcomes for 160,000 postmenopausal women, some of them over the course of 15 years. One aspect of the WHI -- the hormone trial – had been expected to last about eight years, but in June 2002, one arm of the trial was stopped prematurely.4
On July 9, 2022, Jaques Rossouw, M.D., an epidemiologist who was the acting director of the Women’s Health Initiative, shared alarming results from the trial.5
Women on the estrogen plus progestin therapy had a 26% higher incidence of breast cancer than those taking a placebo, he said. “These findings are the first confirmation from a rigorous clinical trial that taking estrogen plus progestin increases the risk of breast cancer.” The findings also showed a 22% increase in total cardiovascular disease, with a 29% increase in heart attacks, a 41% increase in strokes, and a doubling of the rate of blood clots in lungs.
He provided some perspective during the press conference, but the damage was done.
“These data are bound to sound frightening to women. So let me be sure you understand their significance. Those data describe the increased risk for an entire population--not the increased risk for an individual woman. The increased risk of breast cancer for each woman in the WHI study who was taking the estrogen plus progestin therapy, for instance, was actually very small. It was less than a tenth of 1 percent per year.
“The point is that, while we want to get the word out to women and their doctors that long-term use of this therapy could be harmful, women should not conclude that they will develop breast cancer or have a heart attack or stroke if they've taken this medication and, even in those who do suffer one of these diseases, the condition may not be due to the therapy.”
Recovery
The media attention led to fear and confusion and a dramatic reduction in prescriptions for menopausal hormone therapy. But subsequent studies from the Women's Health Initiative and others clearly showed that younger women and those close to menopause had a very beneficial risk-to-benefit ratio.6 Indeed, the results showed similar protective effects for coronary disease and a reduction in mortality that had been shown in earlier studies, which had also focused on younger symptomatic women. In younger women, the increased number of cases of venous thrombosis and ischemic stroke was low, rendering them “rare” events, using World Health Organization nomenclature. Breast cancer rates were also low and were found to be decreased with estrogen alone. In women receiving estrogen and progestogen for the first time in the WHI, breast cancer rates did not increase significantly for seven years. Other data suggested that other regimens and the use of other progestogens may also be safer.
Today menopausal hormone therapy is on an upward track once again. The ability to have a patch, spray, or gel ensures adherence. The emotional and physical symptoms can be reduced.
In its 2017 Hormone Therapy Position Statement, the North American Menopause Society wrote that hormone therapy remained the most effective treatment for vasomotor symptoms (e.g., hot flashes and nocturnal sweats) and the genitourinary syndrome of menopause, and had been shown to prevent bone loss and fracture.7
In February 2023, the National Health Service announced that women in England could access cheaper hormone replacement therapy for menopause through a new prescription prepayment certificate, enabling them access to a year’s worth of menopause prescription items for the cost of two single prescriptions.8
And a September 2023 study from Sweden found that while the use of menopausal hormone therapy decreased from 2000-2007, it stabilized and then increased from 2017 to 20219
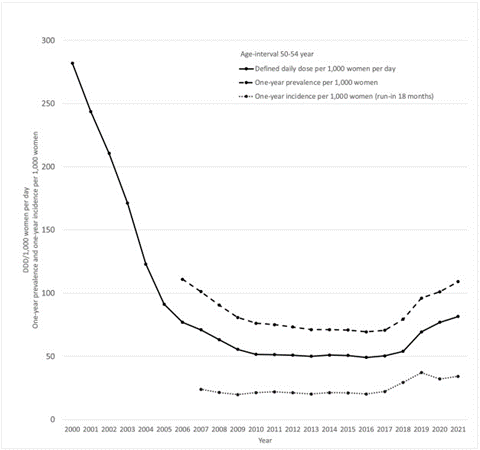
Fig. 1. Menopausal hormone therapy in Sweden, women aged 50–54 years. Volume in Defined Daily Doses (DDD)/1000 women per day, one-year prevalence and incidence (with an 18-month run-in) per 1000 women and year.
Alternative Therapies
Despite the green light conferred to menopausal hormone therapy, alternative menopausal therapies are increasing in popularity.
In 2022, researchers from the University of Wroclaw in Poland reported that almost two decades after the presentation of the first results of the Women’s Health Initiative study, the demand for nonconventional/alternative therapies – e.g., herbalism, acupuncture, yoga, aromatherapy, massage, relaxation, cognitive behavioral modifications, antidepressants, antiepileptics, antihypertensives and regular physical activity – had increased substantially.10
“Satisfaction was found with both conventional and alternative treatments for the relief of menopausal symptoms. The women who used [menopausal hormone therapy] more often reported benefits in sexual life, whereas the women who used alternative methods reported benefits in relation to sexual life, skin condition and physical activity.”
References
- Women Have Been Misled About Menopause, New York Times
- Increased Risk of Endometrial Carcinoma among Users of Conjugated Estrogens, The New England Journal of Medicine
- Women Have Been Misled About Menopause, New York Times
- Ibid
- Release of the Results of the Estrogen Plus Progestin Trial of the Women’s Health Initiative: Findings and Implications, Women’s Health Initiative
- Where Are We 10 Years After the Women’s Health Initiative? The Journal of Clinical Endocrinology & Metabolism
- The 2017 hormone therapy position statement of The North American Menopause Society, Menopause
- Hundreds of thousands of women experiencing menopause symptoms to get cheaper HRT, Department of Health and Social Care, UK
- Trends in the incidence, prevalence and sales volume of menopausal hormone therapy in Sweden from 2000 to 2021, Maturitas
- Prevalence of menopausal hormone therapy and alternative methods, health benefits experienced by peri- and postmenopausal Polish women, Prz Menopauzalny


Share Article